
Isothermal Box: Complete Guide
In the transport of pharmaceutical products, biological samples, or chemical reagents, the cold chain is not an option, it's an absolute obligation. A single degree of deviation can compromise months of research, the viability of a treatment, or the validity of a diagnosis. With current climate challenges, particularly during summer periods when temperatures can reach unexpected heights, mastering the preparation protocol is more critical than ever.
However, the performance of an isothermal packaging, even the most sophisticated one, depends 80% on the rigor of its preparation. Human error remains the weak link in an otherwise optimized logistics chain.
This article is your reference guide to understand the challenges, master the preparation protocol, and choose the right solutions to guarantee compliance and integrity of each temperature-sensitive shipment.

Why is the cold chain so critical for your shipments?
The main concern when shipping under controlled temperature is the risk of temperature excursion, i.e., going outside the required temperature range (for example, +2°C to +8°C). Unlike a simple package, the consequences here are often irreversible:
- Product Loss: Therapeutic proteins, vaccines, or reagents can lose all their efficacy, resulting in a direct financial loss that can amount to thousands of euros.
- Data Invalidation: For clinical trials or research, a cold chain break can render months of work and precious data completely unusable.
- Patient Risk: In the case of medical treatment, a product whose efficacy has been altered can have direct consequences on a patient's health.
Understanding the Dual Regulatory Framework: Cold Chain and Dangerous Goods
For a logistics professional in the life sciences sector, the complexity is twofold. It's not enough to maintain temperature; you must also often comply with dangerous goods transport regulations. The shipper must therefore navigate two regulatory frameworks in parallel:
- Good Distribution Practices (GDP): These standards, derived from the pharmaceutical sector, require proof that the cold chain has been maintained at all stages of transport.
- Dangerous Goods Regulations (IATA, ADR, IMDG): If the transported product (for example, a biological sample) is classified as dangerous goods, the packaging must also be UN certified.

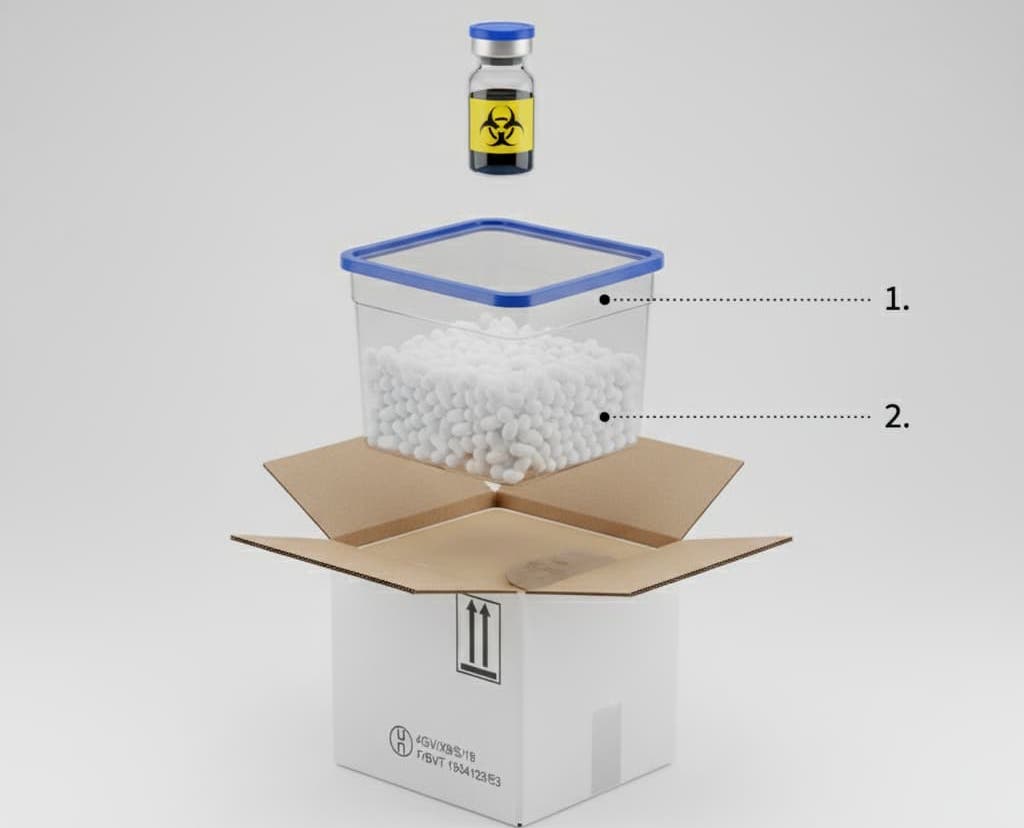
Decoding the Key Standard: UN 3373 and Packaging Instruction P650
The most common use case for packaging that is both isothermal and certified is the transport of Category B biological samples, classified under UN 3373 code. To be compliant, the packaging must respect Packaging Instruction P650, which requires a triple packaging system:
A leak-proof primary receptacle (the tube)
A leak-proof secondary packaging with absorbent
A rigid and resistant outer packaging
Packaging Solutions: The Key to Compliance and Safety
The solution to this dual challenge lies in using a packaging system that has dual competence: it must be both thermally qualified and physically certified.
The importance of "Qualification": The thermal guarantee
A "qualified" packaging has been tested in a climatic chamber according to strict temperature profiles (simulating summer and winter). This qualification gives you assurance that the system can maintain the required temperature for a determined duration (e.g., 96h), even in case of logistics contingencies.

The importance of "Certification": The physical resistance guarantee
UN certification guarantees that the packaging has withstood drop, stacking, and perforation tests. It's your legal assurance that the packaging is suitable for safely containing dangerous materials.
The crucial role of coolants and their preparation
The thermal performance of the entire system depends on the proper use of coolants (eutectic gels). Their pre-conditioning (stabilization at 0°C after removal from the freezer) is a non-negotiable step to avoid freezing the product.
To meet these requirements, discover our complete range of isothermal and certified packaging solutions, designed to guarantee the safety and compliance of your shipments.
FAQ: Your Questions on Isothermal Shipment Preparation
Protocol Rigor, Guarantee of Your Success
The success of a cold chain shipment doesn't rely on luck, but on the combination of a high-performance packaging solution and a preparation protocol applied with absolute rigor. Each step, from stabilizing eutectic gels to final labeling, is an essential link in this safety chain.
By mastering this process, a company doesn't just protect its product; it protects its reputation, guarantees partner trust, and ensures continuity of its research projects or patient care. It's the mark of operational excellence that inspires confidence.
Looking for a certified isothermal solution designed to simplify this protocol and guarantee maximum performance?
Discover our range of qualified packaging and contact our experts to define the solution adapted to your logistics flows.